

科普文章
FcγRIIB交联依赖性激动性抗体LVGN6051(抗CD137)和LVGN7409(抗CD40)的开发机制[1]
1.TNFSF-TNFRSF表达谱和生物学功能
肿瘤坏死因子超家族(TNFSF)和肿瘤坏死因子受体超家族(TNFRSF)分别由19种配体和29种受体组成,包括CD137 (TNFRSF9,4-1BB) 和CD40 (TNFRSF5)[2]。TNFSF和TNFRSF与适应性免疫系统由共同的祖先进化而来[3-5]。进化史表明,TNFSF和TNFRSF成员结构和功能具有高度保守性。在结构上,他们都具有相似的激活下游信号的配体受体三聚体结构。功能上,TNFRSF成员具有某种程度上共性的表达谱和生物学功能,例如,促进T细胞和B细胞的增殖或树突细胞和吞噬细胞的成熟。同时,每一个TNFSF或者TNFRSF家族成员的表达谱,信号网络和对于免疫系统功能的影响都应该有其个性化的区别。例如CD27,CD37和OX40在刺激T细胞对抗肿瘤时分别发挥不同作用。CD27和HVEM表达在激活早期的休眠的T细胞上,而OX40 和CD137信号则出现在激活晚期的T细胞,OX40 和 CD137分别主要影响CD4 T和CD8 T细胞[6]。另一项关于CD27,CD137,OX40 和 GITR对CD8 T细胞细胞因子分泌的影响研究表明,尽管CD137和CD27增加了免疫系统对刺激的敏感性,但是只有CD137能够延长应答反应持续时间,增加细胞因子分泌量,相反,GITR和OX40几乎没有影响[7]。在用自体肿瘤细胞进行的体外肿瘤浸润淋巴细胞(TILs)刺激实验中,与OX40+、PD-1+、CD25+和CD69+ TILs相比,CD137+ TILs上IFN-γ、TNF-α、颗粒酶B、穿孔素和IL-2等效应因子表达量最高,提示CD137+ TILs可能具有最强的抗肿瘤细胞毒性。
2. TNFRSF信号的激活依赖于受体聚集反应
TNFRSF可以被其相应的配体TNFSF严格调控激活。如上所述,TNFRSF和TNFSF成员在TNFRSF-TNFSF相互作用和下游信号激活方面都很保守。TNFRSF受体是1型跨膜蛋白,细长的结构,主要包含3-4个高度保守的“半胱氨酸富集结构域”(CRDs)。TNFSF三聚体主要结合到TNFRSF的第2和第3 CRDs 上,促进有活性的TNFSF3-TNFRSF3复合体的形成(图1)[8-10]。该复合体是TNFRSF信号激活的最小单位。事实上,对于大部分TNFRSF成员来说,仅依靠这种三聚体本身并不足以激活下游的信号通路,需要进一步在TNFSF3-TNFRSF3三聚体的基础上形成多聚体才能完全激活TNFRSFs信号[11]。
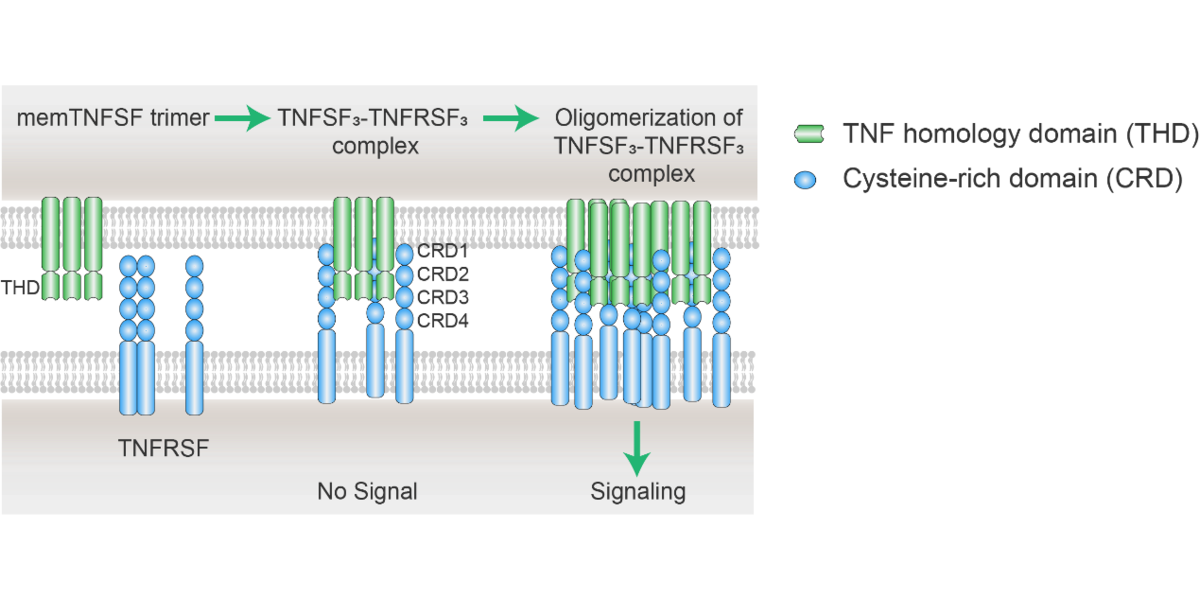
图1.TNFSF3-TNFRSF3复合体的聚集过程[1]。
三聚体膜结合TNFSF通过TNF同源区(TDH)结合到TNFRSF富含半胱氨酸区(CDR),招募三个TNFRSF受体分子,形成TNFSF3-TNFRSF3复合体。然而,对于大部分TNFRSF成员来说,仅依靠这种三聚体本身并不足以激活下游的信号通路(包括CD40、CD137、OX40和GITR),需要进一步在TNFSF3-TNFRSF3三聚体的基础上形成多聚体才能完全激活TNFRSFs信号。
以下是TNFRSF配体与受体之间的激活机制,激动性抗体需要能够促进TNFRSF受体低聚体的形成(图2)。
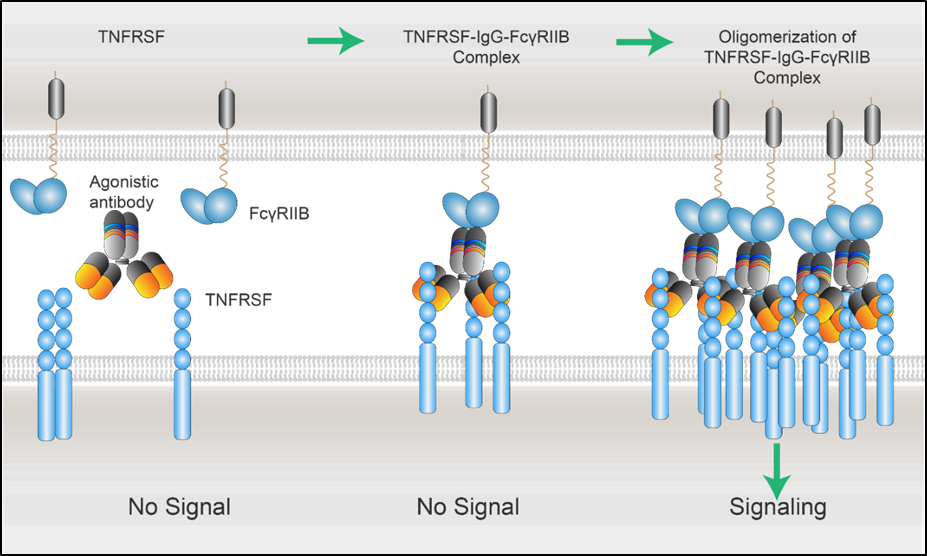
图2 xLinkAb工作模型:FcγRIIB在TNFSFR-IgG-FcγRIIB复合物聚集中的作用[1]。
3. TNFRSF激动性抗体的治疗潜力
许多TNFRSF成员被评估为潜在的新型免疫治疗靶点,特别是考虑到它们在细胞毒性T细胞的激活、克隆扩增和生存方面的重要的直接或间接作用,类似PD-1的调节机制。事实上,20世纪90年代末,靶向TNFRSF的研究在就引起了医学界的兴趣,甚至早于PD-1拮抗剂的开发。除了T细胞共刺激因子CD137外,CD40作为一种罕见的髓系细胞共刺激因子也是肿瘤免疫治疗的重要靶点,有望改善肿瘤微环境,挑战冷肿瘤。如今,有数十种以单克隆或多特异性抗体形式的CD137和CD40激动剂候选药物正在进行临床试验开发、临床扩展策略包括单药或者与免疫检查点抑制剂(如抗CTLA4或抗PD-1/PD-L1抗体)或化疗等标准治疗方案联合治疗[12-15]。表1和表2分别列出了具有代表性的CD137 和 CD40激动性抗体的临床开发情况。
表1. 用于癌症治疗的CD137激动行抗体的临床研究,单药或联合用药


表2. 用于癌症治疗的CD40激动性抗体的临床研究,单药或联合用药


4. 总结
TNFRSF信号通路刺激多种免疫细胞的激活和增殖,包括髓系细胞和T细胞,为癌症免疫治疗的开发提供了潜在靶点。然而,TNFRSF激动性抗体在临床中成功的案例很少,可能是由于早期研发对于TNFRSF下游信号通路依赖受体多聚体的机制不清晰,或者由于剂量限制毒性,临床开发受限于过低的剂量。激活性抗体的激动活性可受到Fab、铰链或Fc区域的影响。通过多年临床前及临床研究积累,Fc-Fc𝛾RIIB相互作用已被确定为激动活性(疗效和毒性)的主要决定因素。FcγRIIB可通过抗体介导TNFRSF受体聚集,激活其下游信号通路,发挥体内抗肿瘤活性的重要作用。体外研究表明,无Fc交联依赖性的强激动性抗体在与靶标结合时表现出激动性,在有FcγRs的情况下成为超级激动剂,导致临床很难克服的毒性问题。相反,在没有Fc交联的情况下,另一类抗TNFRSF抗体可能呈现很弱或无检测到的激动活性,却可在Fc-FcγRIIB交联时表现出强激动活性,这类Fc-FcγRIIB介导的条件性激动剂更有临床开发空间。
经Fc工程化改造的, 与FcγRIIB选择性结合,FcγRIIB交联依赖的TNFRSF激活抗体,掀起了治疗药物临床开发的新浪潮。Fc工程化改造技术应适用于TNFRSF的大部分成员,包括CD40、CD137、OX40、GITR和CD27。此外,交联依赖性可用于开发抗TNFRSF的双特异性或多特异性抗体,其中FcγRIIB可被肿瘤抗原或免疫靶点替代或增加。与这些肿瘤选择性靶点的结合可导致多价肿瘤靶向-抗体-TNFRSF复合物的形成,进而在肿瘤微环境中促进TNFRSF的聚集和下游信号选择性激活。
综上所述,免疫共刺激靶点,特别是TNFRSF成员如CD137和CD40,使得肿瘤免疫疗法仍存在巨大发展空间。从临床疗效、安全性、生产工艺和分子稳定性等方面考虑,IgG结构的单克隆抗体激动剂相比于早研阶段的双抗有其独特的优势。包括礼进生物公司开发的xLinkAb模型在内,进一步探索FcγRIIB交联依赖的Fc工程改造方法成为下一步研究重点[1]。
本文仅作信息分享,不代表礼进生物公司立场和观点,也不作治疗方案推荐和介绍。如有需求,请咨询和联系正规医疗机构。
参考文献:
1.Liu, L., et al., Antibody-Targeted TNFRSF Activation for Cancer Immunotherapy: The Role of FcγRIIB Cross-Linking. Front Pharmacol, 2022. 13: p. 924197.
2.Dostert, C., et al., The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiological Reviews, 2019. 99(1): p. 115-160.
3. Collette, Y., Gilles, A., Pontarotti, P., and Olive, D., A Co-evolution Perspective of the TNFSF and TNFRSF Families in the Immune System. Trends Immunol, 2003. 24 (7), 387–394.
4.Glenney, G.W. and G.D. Wiens, Early Diversification of the TNF Superfamily in Teleosts: Genomic Characterization and Expression Analysis. The Journal of Immunology, 2007. 178(12): p. 7955-7973.
5.Wiens, G.D. and G.W. Glenney, Origin and evolution of TNF and TNF receptor superfamilies. Developmental & Comparative Immunology, 2011. 35(12): p. 1324-1335.
6.Watts, T.H., TNF/TNFR FAMILY MEMBERS IN COSTIMULATION OF T CELL RESPONSES. Annual Review of Immunology, 2005. 23(1): p. 23-68.
7.Nguyen, J., et al., Quantitative contributions of TNF receptor superfamily members to CD8+ T-cell responses. Molecular Systems Biology, 2021. 17(11): p. e10560.
8.Banner, D.W., et al., Crystal structure of the soluble human 55 kd TNF receptor-human TNFβcomplex: Implications for TNF receptor activation. Cell, 1993. 73(3): p. 431-445.
9.Locksley, R.M., N. Killeen, and M.J. Lenardo, The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell, 2001. 104(4): p. 487-501.
10.Compaan, D.M. and S.G. Hymowitz, The Crystal Structure of the Costimulatory OX40-OX40L Complex. Structure, 2006. 14(8): p. 1321-1330.
11. Wajant, H., Principles of antibody-mediated TNF receptor activation. Cell Death & Differentiation, 2015. 22(11): p. 1727-1741.
12.Croft, M., C.A. Benedict, and C.F. Ware, Clinical targeting of the TNF and TNFR superfamilies. Nature Reviews Drug Discovery, 2013. 12(2): p. 147-168.
13.Assal, A., et al., Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy, 2015. 7(11): p. 1169-1186.
14.Burugu, S., A.R. Dancsok, and T.O. Nielsen, Emerging targets in cancer immunotherapy. Seminars in Cancer Biology, 2018. 52: p. 39-52.
15.Lee, D.H., Update of early phase clinical trials in cancer immunotherapy. BMB Rep, 2021. 54(1): p. 70-88.
16.Segal, N.H., et al., Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin Cancer Res, 2017. 23(8): p. 1929-1936.
17.Sznol, M., et al., Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). Journal of Clinical Oncology, 2008. 26(15_suppl): p. 3007-3007.
18.Post, T.A., SITC 2016: Phase I/II Data Combining Urelumab With Nivolumab Suggest Increased Antitumor Effect in Patients With Melanoma. 2016.
19.Segal, N.H., et al., Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin Cancer Res, 2018. 24(8): p. 1816-1823.
20.Tolcher, A.W., et al., Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin Cancer Res, 2017. 23(18): p. 5349-5357.
21.Inc., A., Annual Report. Agenus Inc., 2021. https://investor.agenusbio.com/static-files/2f3ed22a-bd00-4e12-a78c-205169623f82.
22.Tolcher, A.W., et al., Initial findings of the first-in-human phase I study of AGEN2373, a conditionally active CD137 agonist antibody, in patients (pts) with advanced solid tumors. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 2634-2634.
23.release, C.n., Alligator Bioscience presents positive Phase I data at ASCO for its 4-1BB agonist drug candidate ATOR-1017. Alligator, 2021.
24.Ullenhag, G.J., et al., A first-in-human, multicenter, open-label, phase 1 study of ATOR-1017, a 4-1BB antibody, in patients with advanced solid malignancies. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 2646-2646.
25.Compass, Compass Therapeutics Presentation., 2022. https://investors.compasstherapeutics.com/static-files/c55cc1d1-9559-4a76-8ec0-863ef49bbb3a.
26. Compass, Annual Report. Compass, 2022. https://investors.compasstherapeutics.com/static-files/85e7dfff-af0a-4a01-b251-ffce313585cc.
27.Zhang, L., Identification of a predictive biomarker and two pharmacodynamic biomarkers to ADG106 treatment, a novel anti-CD137 agonist antibody, in phase I clinical trials. Journal of Clinical Oncology, 2021. 39(15_suppl): p. e14505-e14505.
28. Adagene, Compary presentation Adagene, 2022. https://investor.adagene.com/static-files/3d44a810-1d77-4bc5-8641-af8a771d45c9.
29.1025MO - First-in-human (FIH) phase I study of RO7122290 (RO), a novel FAP-targeted 4-1BB agonist, administered as single agent and in combination with atezolizumab (ATZ) to patients with advanced solid tumours. ESMO, 2020.
30.Annual Report. Inhibrx,Elpiscience, 2021. https://app.quotemedia.com/data/downloadFiling?webmasterId=101533&ref=116503700&type=PDF&formType=10-K&dateFiled=2022-02-28&cik=0001739614&CK=1739614&symbol=0001739614&companyName=Inhibrx%2C+Inc.
31.BioNtech Corporate Presentation. BioNtech, 2022. https://investors.biontech.de/static-files/2953cd1e-d45e-4597-8d6c-bc2e48269598.
32.Garralda, E., et al., 412 First-in-human phase I/IIa trial to evaluate the safety and initial clinical activity of DuoBody®-PD-L1×4–1BB (GEN1046) in patients with advanced solid tumors. Journal for ImmunoTherapy of Cancer, 2020. 8(Suppl 3): p. A250-A251.
33.BioNTech Innovation BioNTech, 2022 https://investors.biontech.de/static-files/edee73bd-1620-4f51-a419-7ce7782ffa0f.
34. I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Six Months Ended June 30, 2022. Corporate updates, 2022. https://www.i-mabbiopharma.com/i-mab-provides-business-and-corporate-updates-and-reports-financial-results-for-the-six-months-ended-june-30-2022/.
35.Sarina Piha-Paul, M., Phase 1 Dose Escalation Study of PRS-343, a HER2/4-1BB Bispecific Molecule, in Patients with HER2+ Malignancies AACR, 2021.
36.Sarina Piha-Paul1, J.B., Anthony Tolcher3, Sara Hurvitz4, , et al., A Phase 1 Dose Escalation Study of PRS-343, a HER2/4-1BB Bispecific Molecule, in Patients with HER2-positive Malignancies. ESMO, 2020.
37.Annual Report. Pieris, 2022. https://www.pieris.com/investors/sec-filings/all-sec-filings/content/0001583648-22-000065/0001583648-22-000065.pdf.
38.Grilley-Olson, J.E., et al., SEA-CD40, a non-fucosylated CD40 agonist: Interim results from a phase 1 study in advanced solid tumors. Journal of Clinical Oncology, 2018. 36(15_suppl): p. 3093-3093.
39.Bajor, D.L., et al., Preliminary results of a phase 1 study of sea-CD40, gemcitabine, nab-paclitaxel, and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (PDAC). Journal of Clinical Oncology, 2022. 40(4_suppl): p. 559-559.
40.Vonderheide, R.H., et al., Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol, 2007. 25(7): p. 876-83.
41.Vonderheide, R.H. and M.J. Glennie, Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res, 2013. 19(5): p. 1035-43.
42.Beatty, G.L., et al., A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res, 2013. 19(22): p. 6286-95.
43.Nowak, A.K., et al., A phase 1b clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann Oncol, 2015. 26(12): p. 2483-90.
44.ajor, D.L., et al., Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology, 2018. 7(10): p. e1468956.
45.Barlesi, F., et al., 291 Phase Ib study of selicrelumab (CD40 agonist) in combination with atezolizumab (anti-PD-L1) in patients with advanced solid tumors. Journal for ImmunoTherapy of Cancer, 2020. 8(Suppl 3): p. A178-A178.
46.Calvo, E., et al., A phase I study to assess safety, pharmacokinetics (PK), and pharmacodynamics (PD) of JNJ-64457107, a CD40 agonistic monoclonal antibody, in patients (pts) with advanced solid tumors. Journal of Clinical Oncology, 2019. 37(15_suppl): p. 2527-2527.
47. Corporate presentation Apexigen, 2022. https://ir.apexigen.com/static-files/451a9989-fcaf-4d3e-875f-8dbbae9c0881.
48.Weiss, S., et al., 389 Phase II of CD40 agonistic antibody sotigalimab (APX005M) in combination with nivolumab in subjects with metastatic melanoma with confirmed disease progression on anti-PD-1 therapy. Journal for ImmunoTherapy of Cancer, 2021. 9(Suppl 2): p. A422-A422.
49.Kluger, H., et al., Abstract CT089: Phase Ib/II of CD40 agonistic antibody APX005M in combination with nivolumab (nivo) in subjects with metastatic melanoma (M) or non-small cell lung cancer (NSCLC). Cancer Research, 2019. 79(13_Supplement): p. CT089-CT089.
50.Padrón, L.J., et al., Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nature Medicine, 2022. 28(6): p. 1167-1177.
51.Sanborn, R., et al., 405 CDX1140–01, a phase 1 dose-escalation/expansion study of CDX-1140 alone (Part 1) and in combination with CDX-301 (Part 2) or pembrolizumab (Part 3). Journal for ImmunoTherapy of Cancer, 2020. 8(Suppl 3): p. A246-A246.
52.Company Annual Report. Celldex, 2021. https://ir.celldex.com/static-files/91091415-8d60-43f4-a20a-b1e7a11bbb94.
53.Coward, J., et al., Phase I open-label, dose escalation of YH003, an anti-CD40 monoclonal antibody in combination with toripalimab (anti-PD-1 mAb) in patients with advanced solid tumors. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 2580-2580.
54.Biocytogen/Eucure Biopharma's YH003 (Anti-CD40 Monoclonal Antibody) Approved for Phase II Multi-Regional Clinical Trial by China National Medical Products Administration. Company release, 2021. https://www.eucure.com/en/detail/450.html.







 沪公网安备 31011502015333号
沪公网安备 31011502015333号