

科普文章
xLinkMsAb,新一代T细胞接合剂(TCE)激动性抗体
1. 双特异性抗体未来市场预测
双特异性抗体(bsAb)是一类能够识别两个表位或抗原的抗体。大多数bsAb是双特异性T细胞接合剂 (T-cell-engager ,TCE),设计用于重定向激活表达CD3的细胞毒性T细胞(cytotoxic T cells, CTLs)到恶性肿瘤上的特定靶点,以达到杀伤肿瘤的目的。目前有6种已获批准的CD3双特异性TCE,超过150种候选TCEs正在临床开发(表1)[1],体现了CD3 TCE在肿瘤治疗中的重要作用(表1)和预期的巨大市场规模(图1)。
在此处添加文本段落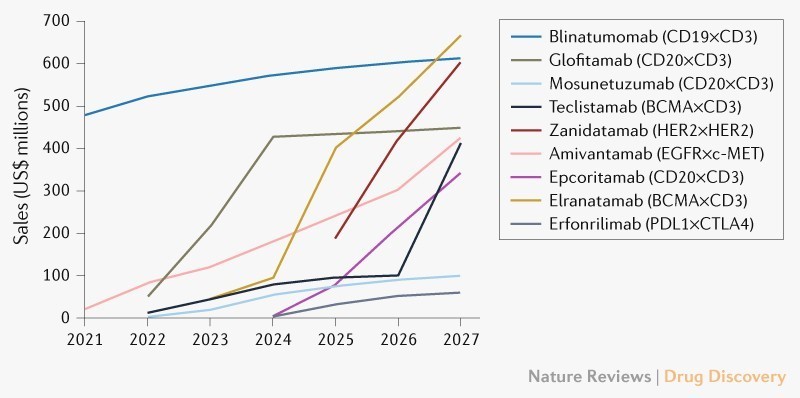
图1.TCEs的市场预测[2]。全球bsAb肿瘤市场预计将迅速扩大,预计到2027年销售额将接近37亿美元(图1) [2]。
在这些疗法中,由于复发/难治性多发性骨髓瘤患者数量逐年升高,预计elranatamab将成为最畅销的TCE (2027年为6.65亿美元)。Tecvayli (teclistamab)的销售额预计将紧随其后 (2027年为4.1亿美元)。
blinatumomab的销售额将保持较高水平并趋于平稳(2027年为6.15亿美元),主要是因为blinatumomab已被纳入急性淋巴细胞白血病的标准治疗方案。
据估计,到2027年,靶向CD20×CD3的药物在治疗B细胞非霍奇金淋巴瘤(NHL)中的销售将占TCE市场份额的近25%。预计Glofitamab将是三种CD20×CD3 TCE中最畅销的(4.5亿美元),其次是epcoritamab (3.45亿美元)。这两种药物的适应症还包括弥漫大B 细胞淋巴瘤(DLBCL),基于该适应症的患者基数大,销售额将进一步提升。受到治疗机会的减少和现有药物竞争的限制,Mosunetuzumab预计将在滤泡性淋巴瘤领域获得1亿美元的销售额。这三种药物也必将面临已经批准和正在出现的靶向疗法和CAR-T细胞疗法的竞争。
2. TCE相较于CART的优势
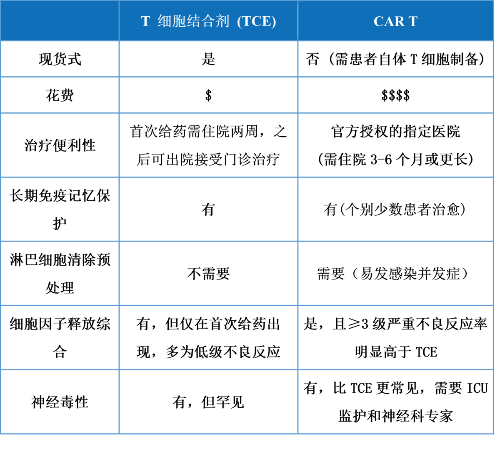
嵌合抗原受体(CAR) T细胞是一种利用自体工程改造T细胞发挥抗肿瘤作用的细胞疗法。值得一提的是,大多数市场批准的CAR T产品具有4-1BB共刺激结构域,这也是礼进生物构建xLinkMsAb TA/CD3/4-1BB多抗的科学依据之一。相较于CART,TCE主要有两大明显优势,1)更安全的“现货”产品:提供了一种可以通过起始低剂量递增方式有效控制细胞因子释放综合征(cytokine release syndrome, CRS)不良反应的高安全性的现货式产品;2)更具有普惠性:如果考虑到CART细胞生产、物流、住院治疗和不良事件处理等方面,CART带来的医疗和经济负担会比TCE要大很多(表2/3)[3]。然而,目前临床阶段或已上市TCE仍伴随有常见不良事件包括CRS (尽管大部分为低级别),以及3级或4级严重血液毒性(血小板减少、中性粒细胞减少、淋巴细胞减少和贫血等)(表2)。另外,TCE或者CART均未在实体瘤领域获得突破。因此,仍有开发具有更优的安全性、更长的生存获益和更低成本的新一代TCE的迫切需要和市场空间。
表1.已批准的和临床晚期TCE
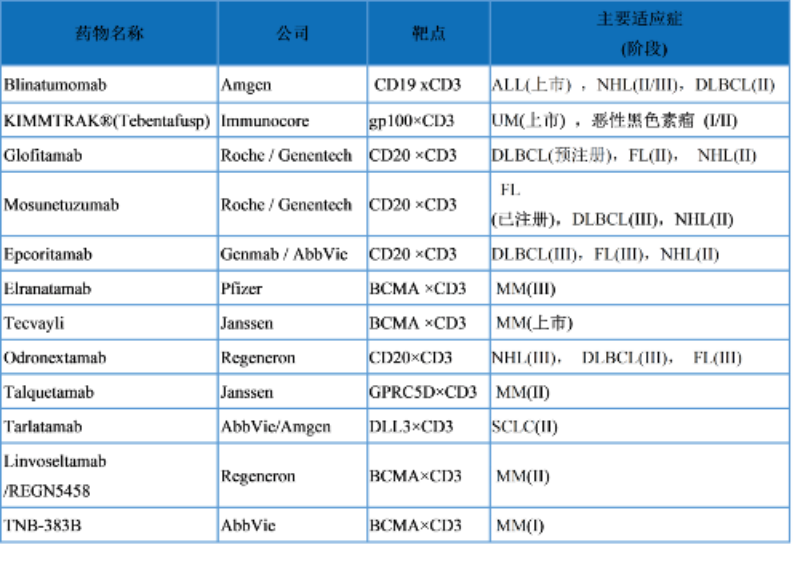
ALL:急性B淋巴细胞白血病 NHL: 非霍奇金淋巴瘤 DLBCL:弥漫大B淋巴瘤 FL:滤泡性淋巴瘤 MM:多发性骨髓瘤 SCLC:小细胞肺癌
表2. 上市或临床后期开发阶段TCE的不良反应和有效性

ORR:客观缓解率 CR:完全缓解 VGPR:非常好的部分缓解 OS:总生存期
表3. TCE与CART的比较
3. 新一代TCE
4-1BB (CD137)是一种可诱导的表达于活化T细胞和自然杀伤(NK)细胞上的共刺激受体。4-1BB配体结合到T细胞表面触发信号级联反应,促使抗凋亡分子、细胞因子的上调,增强细胞毒性等效应反应[16]。4-1BB激动剂抗体对于CD3 TCE杀瘤功能的增强效应在多个临床前研究中得以证明。如图2所示,CD20 CD3 T细胞双特异性抗体glofitamab诱导特异性T细胞活化,在复发/难治性非霍奇金淋巴瘤患者中显示出显著的单药活性[17]。而RO7227166是一种靶向CD19的4-1BBL (CD137)共刺激激动剂,在与glofitamab联用时显示出协同抗肿瘤活性[18]。RO7227166的预期作用机制(MoA)是递送共刺激信号2 (4-1BB),从而在T细胞双特异性抗体(例如glofitamab)激活信号1(CD3)后,增强肿瘤浸润T细胞或NK细胞的效应功能。通过更直接的T细胞-靶细胞结合,进行共刺激激活,提供了一种高效的现货式的免疫治疗手段。RO7227166目前正在一项I期、开放标签、剂量递增研究BP41072 (NCT04077723)中探索与glofitamab联用的治疗方案。
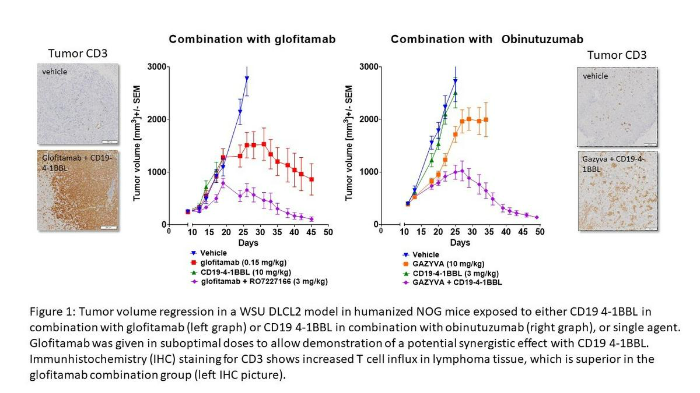
图2:在接受CD19 4-1BBL联合glofitamab或单药治疗的人源化NOG小鼠中,WSU DLCL2模型中的肿瘤体积退缩。为了证明与CD19 4-1BBL的潜在协同作用,我们以次优剂量给予了Glofitamab。CD3的免疫组织化学(IHC)染色显示淋巴瘤组织中T细胞内流增加,这一情况在glofitamab联合治疗组中优于对照组(IHC图) [18]。
4. xLinkMsAb:多特异性肿瘤靶向TA/CD3/4-1BB激动性抗体
xLinkMsAb是一种类IgG结构的多特异性激动性抗体,由CD3激活结构域、4-1BB激活结构域、肿瘤抗原靶向结合域和沉默Fc端组成(图3)。4-1BB是可诱导的由活化的T细胞或NK细胞表达的共刺激分子。通过与肿瘤抗原的结合,CD3和4-1BB激活结构域可以刺激T细胞,特别是通过4-1BB的共刺激信号增强T细胞的细胞毒性,直接杀伤表达肿瘤抗原或周边的肿瘤细胞。
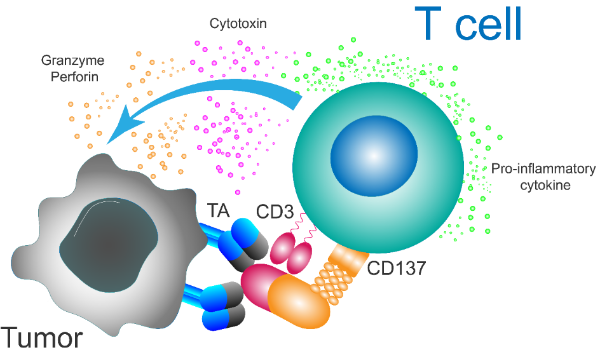
图3:xLinkMsAb:肿瘤抗原依赖的CD3/4-1BB共激活TCE
xLinkMsAb平台旨在通过限制在外周组织的暴露和活性,同时专注于增强肿瘤中表达4-1BB的CD8效应T细胞的抗肿瘤细胞毒性,从而增加治疗窗口。除了4-1BB共刺激靶点,我们的xLinkMsAb平台还包含其它多个共刺激/抑制检查点结合位点的不同组合方式,借助于不同免疫调节靶点间的协同效应,利用系统生物学的概念,从整体设计治疗窗口更佳的新一代肿瘤免疫功能增强TCE。同时,我们正在设计针对不同血液或实体肿瘤的肿瘤抗原的系列xLinkMsAb TCE。目前,我们的候选药物分子已通过一系列的体外和体内实验中验证了xLinkMsAb平台具有严格肿瘤抗原依赖的CD3和4-1BB激活活性,更优的肿瘤细胞毒性,以及比对照双特异性TCE更强的抗肿瘤活性。
此外,得益于我们的多特异性T细胞接合剂(TCE) 的类IgG结构,在动物体内已验证了其具有类似单抗的PK谱,表明xLinkMsAb很可能在人体中表现出良好的药物代谢谱。
综上所述,本研究成功制备了肿瘤抗原依赖性的同时激活CD3和4-1BB的多特异性TCE,其有效性和安全性均优于对照的双特异性TCE,并且具有类似单克隆抗体的PK特征,为提高T细胞接合剂抗体的临床应用,提供了一种独特思路,目前已有候选分子进入后期工艺开发。我们正在积极寻找合作伙伴,请随时通过我们的网站www.lyvgen.com或邮件bd@lyvgen.com联系我们。
本文仅作信息分享,不代表礼进生物公司立场和观点,也不作治疗方案推荐和介绍。如有需求,请咨询和联系正规医疗机构。
参考文献:
1. Wang, S., et al., The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol Med, 2021. 13(9): p. e14291.
2. Esfandiari, A., S. Cassidy, and R.M. Webster, Bispecific antibodies in oncology. Nat Rev Drug Discov, 2022. 21(6): p. 411-412.
3. Subklewe, M., BiTEs better than CAR T cells. Blood Adv, 2021. 5(2): p. 607-612.
4. Kantarjian, H., et al., Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med, 2017. 376(9): p. 836-847.
5. Nathan, P., et al., Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med, 2021. 385(13): p. 1196-1206.
6. Michael Dickinson, C.C.-S., Franck Morschhauser, Emmanuel Bachy, Paolo Corradini, Gloria Iacoboni, Cyrus Khan, Thomasz Wróbel, Fritz Offner, Marek Trněný, Shang-Ju Wu, Guillaume Cartron, Mark Hertzberg, Anna Sureda, David Perez-Callejo, Linda Lundberg, James Relf, Emma Clark, Kathryn Humphrey, Martin Hutchings, GLOFITAMAB INDUCES DURABLE COMPLETE REMISSIONS AND HAS FAVORABLE SAFETY IN PATIENTS WITH RELAPSED/REFRACTORY DIFFUSE LARGE B-CELL LYMPHOMA AND ≥2 PRIOR THERAPIES: PIVOTAL PHASE II EXPANSION RESULTS. EHA, 2022.
7. Budde, L.E., et al., Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. The Lancet Oncology, 2022. 23(8): p. 1055-1065.
8. Catherine Thieblemont, T.P., Herve Ghesquieres, Chan Y. Cheah, Michael Roost Clausen, David Cunningham, Young Rok Do, Tatyana Feldman, Robin Gasiorowski, Wojciech Jurczak, Tae Min Kim, David John Lewis, Marjolein van der Poel, Michelle Limei Poon, Thomas Doerr, Nurgul Kilavuz, Menghui Chen, Mariana Sacchi, Brian Elliott, Martin Hutchings, Pieternella Lugtenburg, PRIMARY RESULTS OF SUBCUTANEOUS EPCORITAMAB DOSE EXPANSION IN PATIENTS WITH RELAPSED OR REFRACTORY LARGE B-CELL LYMPHOMA: A PHASE 2 STUDY. EHA, 2022.
9. Jakubowiak, A.J., et al., Elranatamab, a BCMA-targeted T-cell redirecting immunotherapy, for patients with relapsed or refractory multiple myeloma: Updated results from MagnetisMM-1. Journal of Clinical Oncology, 2022. 40(16_suppl): p. 8014-8014.
10. Moreau, P., et al., Teclistamab in Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine, 2022. 387(6): p. 495-505.
11. Bannerji, R., et al., Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. The Lancet Haematology, 2022. 9(5): p. e327-e339.
12. Minnema, M.C., et al., Efficacy and safety of talquetamab, a G protein-coupled receptor family C group 5 member D x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM): Updated results from MonumenTAL-1. Journal of Clinical Oncology, 2022. 40(16_suppl): p. 8015-8015.
13. AMGEN, Phase 1 Tarlatamab Study Showed Encouraging Antitumor Activity With Median Duration of Response of 13 Months in Small Cell Lung Cancer. Press Releases, 2022.
14. Jeffrey A Zonder, J.R., Naresh Bumma, Jason Brayer, James E Hoffman, William I Bensinger, Ka Lung Wu, Linzhi Xu, Dhruti Chokshi, Anita Boyapati, Damien Cronier, Yariv Houvras, Karen Rodriguez Lorenc, Glenn S Kroog, Madhav V Dhodapkar, Suzanne Lentzsch, Dennis Cooper, Sundar Jagannath, EARLY, DEEP, AND DURABLE RESPONSES, AND LOW RATES OF CRS WITH REGN5458, A BCMAXCD3 BISPECIFIC ANTIBODY, IN A PHASE 1/2 FIRST-IN-HUMAN STUDY IN PATIENTS WITH RELAPSED/REFRACTORY MULTIPLE MYELOMA. EHA, 2022.
15. Kumar, S., et al., A Phase 1 First-in-Human Study of Tnb-383B, a BCMA x CD3 Bispecific T-Cell Redirecting Antibody, in Patients with Relapsed/Refractory Multiple Myeloma. Blood, 2021. 138(Supplement 1): p. 900-900.
16. Chester, C., et al., Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood, 2018. 131(1): p. 49-57.
17. Hutchings, M., et al., Glofitamab step-up dosing induces high response rates in patients with hard-to-treat refractory or relapsed non-Hodgkin lymphoma. Blood, 2020. 136: p. 46-48.
18. Hutchings, M., et al., Phase 1 Study of CD19 Targeted 4-1BBL Costimulatory Agonist to Enhance T Cell (Glofitamab Combination) or NK Cell Effector Function (Obinutuzumab Combination) in Relapsed/Refractory B Cell Lymphoma. Blood, 2020. 136: p. 16-17.
19. Kegyes, D., et al., Patient selection for CAR T or BiTE therapy in multiple myeloma: Which treatment for each patient? J Hematol Oncol, 2022. 15(1): p. 78.







 沪公网安备 31011502015333号
沪公网安备 31011502015333号